EBOVIR
Your Partner in Precision Medicine
For Better Future
From McGill University to Global Precision Medicine Pioneer
About Us
Ebovir Biotechnologie Inc. is a biotech company focused on AI-powered precision medicine. Incubated at McGill University, Ebovir’s precision medicine services are already supporting over 150 high-end health and chronic disease management centers, family practices, OB/GYN and physical exam clinics, and dental clinics across Canada, forming a robust and growing clinical partner network.
Through Whole Genome Sequencing (WGS) and early cancer screening, we empower physicians and healthcare providers to identify pathogenic genetic variants and pinpoint actionable targets associated with cancer, metabolic disorders, rare diseases, and more. This enables more informed prevention strategies, targeted therapeutics, and long-term personalized care planning.
With our state-of-the-art BSL-certified lab in Montreal, a leading AI-powered genomic analysis platform, and a global clinical research network, Ebovir is advancing a new era of integrated healthcare built on the synergy of AI + Genomics + Cell Therapy—turning your unique genetic blueprint into a powerful tool for lifelong health.
Viral Basic Research and Novel Biopharma Therapies Against Viruses

Retrovirus Production And Titration
Retrovirus production and titration are essential steps in the study of retroviruses and their replication. The following steps can be used to produce and titrate retroviruses:
Evaluation of Small Molecules
The evaluation of antiviral activity of small molecules involves testing the ability of these molecules to inhibit the replication of viruses in vitro and in vivo. The following steps can be used to evaluate the antiviral activity of small molecules:

Evaluation Of Antiviral Activity Of Nature Products
The evaluation of antiviral activity of natural products involves testing the ability of these products to inhibit the replication of viruses in vitro and in vivo. The following steps can be used to evaluate the antiviral activity of natural products:
Evaluation Of Antiviral Immune Modulators
The evaluation of antiviral immune modulators involves the assessment of their efficacy, safety, and overall impact on viral infections. This evaluation typically involves the following steps:

Evaluation Of Biologics
The evaluation of viral biologics, also known as viral vaccines or antiviral drugs, involves the assessment of their efficacy, safety, and overall impact on viral infections. This evaluation typically involves the following steps:

Evaluation Of Antiviral Therapeutic Antibodies
The evaluation of antiviral therapeutic antibodies involves the assessment of their efficacy, safety, and overall impact on viral infections. This evaluation typically involves the following steps:

Clinical Trials Consultant
Clinical trials are a crucial step in the development of new treatments and medicines. These trials are designed to evaluate the safety, efficacy, and effectiveness of a new drug or therapy in a controlled and supervised environment..
283
Laboratories in 100+ states
283
Laboratory specialists
283
Material collection points
283
Laboratories in 100+ states
Lorem Ipsum
Lorem Ipsum
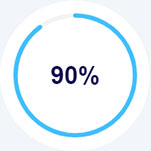
Lorem Ipsum
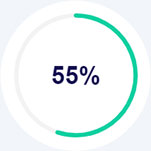
Lorem Ipsum


